About the 24R1 Release
Pre-Release Date: March 25, 2024 | CDB Pre-Release Date: April 1, 2024 | Release Date: April 12 & 19, 2024This page can help you understand the schedule for the coming release and the list of features included. Dates are subject to change.
Important Dates
24R1 Pre-Release Begins
- Pre-release vaults available: Your Veeva Project Manager will contact you with login credentials.
- Validation documents available:
- Validation Project Plan
- IOQ Protocol
- Business Requirements Documents
- Validation Impact Assessment
24R1 Pre-release for CDB Begins
Pre-release environments available for Vault CDB. Contact your Veeva Project Manager for details.
24R1 Release, Part A
- 24R1 released to pre-release (VV1-37) and limited release (VV1-17) vaults
24R1 Release, Part B
- 24R1 released to all general release vaults.
- Updated training available on Veeva Learning
24R1 Pre-Release Ends
We recommend that you no longer use your 24R1 pre-release vaults after this date. Even though your pre-release vault is still active, we advise waiting for the next pre-release period (24R1) to begin. Contact your Veeva Project Manager for details.
Feature Information
- What’s New in 24R1 (Updated April 19) provides detailed explanations of each feature.
- 24R1 Feature Enablement Details (Updated April 19) includes information on how new features are enabled.
Pre-Release Information
- Pre-Release FAQ answers common questions about “pre-release”.
- Known Issues in 24R1 Pre-Release (Updated April 19) lists known issues in the 24R1 pre-release. This page also lists the release notes for any maintenance releases applied to pre-release.
Release Information
- 24R1 Release Impact Assessment (Updated April 30) analyzes the impact of new features. If you need an editable version, we also provide an MS Excel™ version.
- 24R1 Migration Vault Release Impact Assessment (Published February 26) analyzes the impact of new features. If you need an editable version, we also provide an MS Excel™ version.
- 24R1 Supplemental Vault Release Impact Assessment (Published March 1) lists Vault Platform features in the 24R1 release that can impact CDMS vaults.
- 24R1 Fixed Issues (Updated April 19) documents issues that affected previous versions or pre-release and are fixed in 24R1.
- 24R1 Known Issues (Updated April 19) documents issues introduced in 24R1 which are not yet fixed.
- 24R1 Maintenance Releases (Available with first maintenance release) documents fixes for issues that are affecting customers in production environments.
For details about new features in the Vault Platform in 24R1, see the Vault Release Notes .
Notifications Opt-In
As a valued member of our community, providing you with control over the communications you receive from Veeva is important to us. Please allow us to send you pre-release environment notifications and other industry related information. Contact your vault’s administrator to verify that the Product Announcements checkbox is selected for your user profile.
Announcements
EDC Clinical Reporting
EDC Clinical Reporting is a new CDMS application in 24R1. EDC Clinical Reporting provides access to EDC form data for studies not using CDB Workbench. This application is automatically added to Studies not using the CDB Workbench application. Contact your Veeva Services representative for details.
Pre-release: EDC Clinical Reporting is not available in pre-release. This application will be available with the 24R1 general release.
VeevaID & EDC
Veeva EDC onboarding for site users with VeevaIDs will begin after the release of 24R2 in August.
After the 24R2 release, new Veeva EDC customers starting their first studies will start using VeevaID immediately for their site users. Existing customers with active studies will work with Veeva to schedule an all at once migration of site users prior to August 2025.
Sponsor SSO will also be supported. If configured by a sponsor, the Sponsor SSO Option will allow the site user to choose to login to a Veeva application with either their SSO ID or their VeevaID, choosing the ID that is easiest and makes the most sense for them at that time. Once logged in, the experience will be the same regardless of which ID is used. The Sponsor SSO Option is planned for availability with the 24R3 release in December 2024.
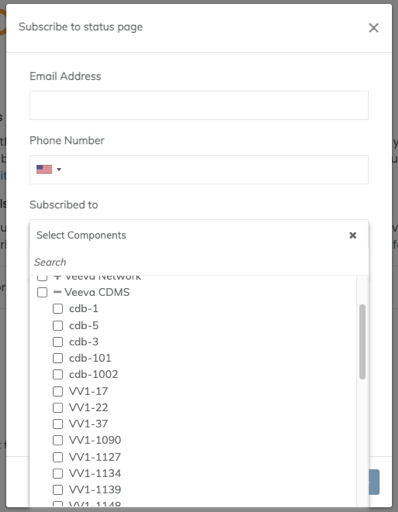
Service Availability Notifications Moving Back to Veeva Trust
Effective immediately, the EDC Service Availability notification subscription has moved back to Veeva Trust. This is being done to ensure that all users can independently subscribe to receive these availability notifications, and to be consistent with CDB Service Availability notification management.
If you previously signed up for notifications on Veeva Trust , that subscription will remain. Otherwise, click the Subscribe link at the top right of the page and complete the Subscribe dialog. You can select the products and PODs for which you’d like to receive notifications.
To support this transition we will utilize both the Vault Opt-in and the Veeva Trust subscription to generate these notifications for the next 2 weeks.
Background
Last summer, subscription to EDC Service Availability Notifications was moved from Veeva Trust and managed through Vault in the EDC System Tools User record. This was done for consistency with other Vault Products. However, we received feedback that users have missed important service availability notifications because they cannot enable this setting independently. The move back to using Veeva Trust will address this concern.
Product Announcement Emails
Product Announcement Emails are those generated to inform and educate about upcoming product updates, releases, etc. Opt in for this type of communication will still be managed using the Product Announcement Emails setting in Vault. Users should contact their Sponsor or User Administrator if they’d like to receive these emails.
Vault Owners and User Administrators will still see the Service Availability Notifications field, but it does not impact the Veeva Trust email sign-ups or notifications.
Data and Definition Export Job Retirement
The Data and Definition Export job is now deprecated and any existing scheduled jobs will be removed. We recommend using the Study Data Extract instead.